Origin of Ciprofloxacin
The quinolones are a class of synthetic antimicrobial agents which have been in use since 1964. Ciprofloxacin is a synthetic fluoropiperazinyl quinolone, and falls into the group of fluorinated 4–quinolones (including norfloxacin and ofloxacin) which have a wide spectrum of antimicrobial activity. Ciprofloxacin has a 6–fluoro substituent that produces enhanced antibacterial toxicity against both Gram–positive and particularly Gram–negative micro-organisms.
The initial compound produced was flumequine, which contrasted with its precursors mainly in its action against Pseudomonas aeruginosa. In addition, it was already known that removal of the piromidic acid substituent at the 7-position and replacement by piperazine to give pipemidic acid produced greater activity against enterobacteria and P. aeruginosa. This was exploited to synthesize a series of 6–fluoro–7–piperazinyl–4-quinolones, of which there are three groups. The second group is comprised of the large and significant group of 6–fluoro-7-piperazinyl compounds, to which ciprofloxacin belongs.
Production of Ciprofloxacin
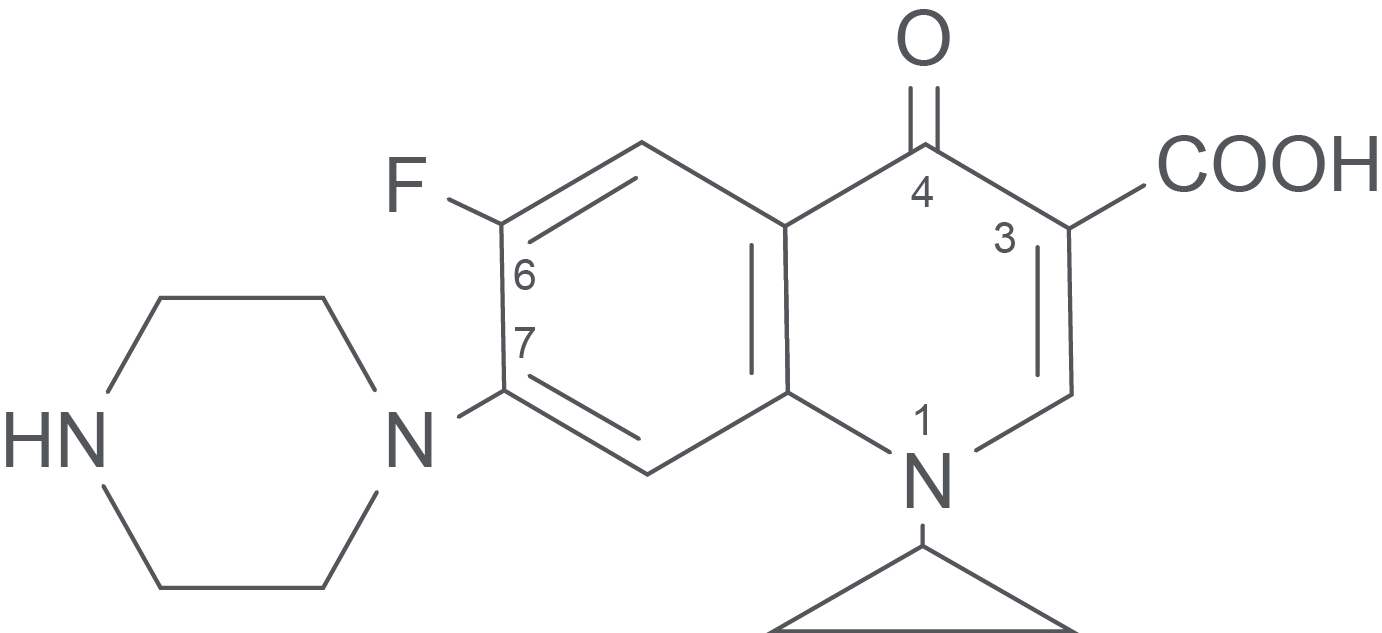
Ciprofloxacin hydrochloride (Figure 1) is prepared by firstly forming the imine of 3–chloro–4-fluoroaniline through condensation with diethyl ethoxymethylenemalonate. This imine is then thermally cyclized to form ethyl 7–chloro–6–fluoro–4-hydroxyquinoline–3–carboxylate. N–alkylation with cyclopropyl iodide, then nucleophilic displacement of the chloro group at position 7 by N–methylpiperazine and finally, hydrolysis of the ester, yields the final product.
Figure 1. The Structure of Ciprofloxacin Hydrochloride.
Mode of Action of Ciprofloxacin in the Human Host
Ciprofloxacin acts by inhibiting the A subunit of the bacterial DNA gyrase enzyme, which is essential for DNA replication, expression and repair. The fluoroquinolones are selective for bacterial DNA gyrase since the eukaryotic cells do not contain bacterial DNA gyrase, and the equivalent mammalian enzyme is structurally dissimilar to DNA gyrase.
Mechanisms of Resistance Against Ciprofloxacin
There are three primary mechanisms by which bacteria may develop resistance to ciprofloxacin as follows:
- A mutation in DNA gyrase (the target) which results in decreased binding of the fluoroquinolone
- An alteration in bacterial membrane permeability (applicable to Gram–negative organisms) resulting in a decreased inflow of ciprofloxacin into the cell (e.g. as a result of changes in the quantity or type of porins in the outer membrane of Gram–negative bacteria), and
- An active efflux mechanism, where ciprofloxacin that passes into the cell is actively expelled.
Ciprofloxacin resistance arises as a result of the selection of mutants which might be prevalent in a population of bacteria. Resistance strategies to ciprofloxacin are coded for by the genes present on the bacterial chromosome, and thus intra– and inter-species relocation of resistance mechanisms to other bacteria does not happen; i.e. resistance to ciprofloxacin has not been found to be linked to plasmids.
Conclusion
Use of the quinolones would most likely have stopped due to the more frequent resistance associated with them, and the notable regularity and severity of side effects concerning the central nervous system (CNS). However, the discovery that the substitution of fluorine onto the 6-position of the molecule altered its characteristics has restored the value/usefulness of this class for treating infections resistant to other drugs, or where a drug with this particular antibacterial spectrum is required.
References:
- Amyes SGB. Magic Bullets, Lost Horizons: The Rise and Fall of Antibiotics. London: Taylor & Francis; 2001.
- Barnas GP. MCW & FMLH Antibiotic Guide. Milwaukee: Medical College of Wisconsin and Froedtert Hospital; 2000.
- Friedman RB. Antimicrobial Use Guidelines. Madison: University of Wisconsin; 1999.
- Gatus BJ. Bacterial susceptibility to Ciprofloxacin: analysis of in vitro data from International and Australian studies. Auckland: Adis International; 1996.
- Gennaro AR, editor. Remington: The Science and Practice of Pharmacy. 20th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
- Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill, 2001.
- Herold M, Gabriel Z, editors. Antibiotics: advances in research, production and clinical use; proceedings of the Congress on Antibiotics held in Prague, 15-19 June, 1964. London: Butterworth & Co; 1966.
- Hugo WB, Russell AD. Pharmaceutical Microbiology. 6th ed. Oxford: Blackwell Science; 1998.
- Iglewski BH. Pseudomonas. Rochester: University of Rochester Medical Centre; 2003.
- Karachalios G, Charalabopoulos K. Biliary Excretion of Antimicrobial Drugs. Chemotherapy 2002;48:280–97.
- Lambert HP, O’Grady FW. Antibiotic and Chemotherapy. 6th ed. London: Churchill Livingstone; 1992.
- Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular Biology of Pseudomonads. Washington DC: ASM Press; 1996.
- Rang HP, Dale MM, Ritter JM. Pharmacology. 4th ed. London: Churchill Livingstone; 1999.
- Ristuccia AM, Cunha BA. Antimicrobial Therapy. New York: Raven Press; 1984.
- Smejkal P. Antibiotics and Chemotherapeutics [lecture]. Prague: Dept Infectious and Tropical Diseases, Charles University; 2002.
- The Medical Letter. Handbook of Antimicrobial Therapy. New York: The Medical Letter; 1998.
- Wilson APR, Grüneburg RN. Ciprofloxacin: 10 years of clinical experience. Oxford: Maxim Medical; 1997.
